New insights into the asymmetric Diels–Alder reaction: the endo- and S-selective retro-Diels–Alder reaction†
Abstract
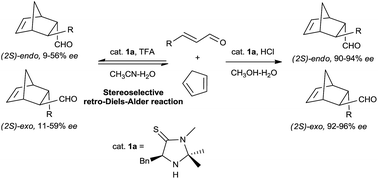
The endo- and S-selective retro-Diels–Alder reactions in an imidazolethione-catalyzed asymmetric Diels–Alder reaction were verified and investigated, and account for the low ee values in a CH3CN–H2O catalytic system. This reverse process could be controlled by forming the dimethyl acetal of the aldehyde product with methanol. Both the exo- and endo-isomers were obtained in the CH3OH–H2O system in high yields with good to excellent enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...