Ionic Diels–Alder reaction of 3-bromofuran toward the highly electron deficient cyclobuteniminium cation: a regio- and stereoselectivity, and molecular mechanism study using DFT
Abstract
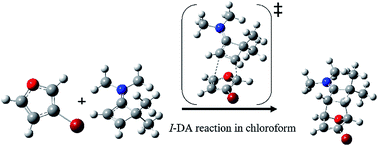
A theoretical study at the MPWB1K/6-311G(d,p) level was performed on the energetic, regio- and stereoselectivity as well as the molecular mechanism of an ionic Diels–Alder (I-DA) reaction of aromatic diene D10, 3-bromofuran, toward highly electron-deficient dienophile Dph11, the cyclobuteniminium cation, in the presence of chloroform. The calculated relative Gibbs free energies indicate that the studied reaction takes place in a complete regio- and stereoselective manner in which the nucleophilic C1 carbon atom of diene D10 is attacked by the strongly electrophilic C6 carbon atom of Dph11 passing through an asynchronous exo transition state TS1x and affording the corresponding cycloadduct CA1x as the unique product in excellent agreement with the experimental outcomes. The relatively high activation Gibbs free energy and slight exergonic nature of this I-DA reaction are related to the aromatic character of D10, estimated using simple isodesmic reactions, which is lost during the cycloaddition. The reasonable regioselectivity presented by the investigated reaction can be explained using calculated electrophilic and nucleophilic Parr functions at the reactive sites of the reagents. On the other hand, a great destabilizing steric repulsion between bulky bromine substitution of D10 and the iminium moiety of Dph11 along the endo stereoselective approach is responsible for the predominance of the exo approach over the endo one. Moreover, an ELF topological analysis of the bonding changes along this I-DA reaction supports a non-concerted two-stage one-step molecular mechanism.

 Please wait while we load your content...
Please wait while we load your content...