Significance enhancement in the conductivity of core shell nanocomposite electrolytes
Abstract
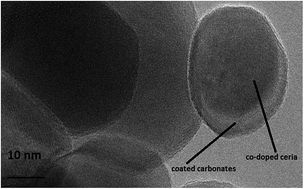
Today, there is great demand of electrolytes with high ionic conductivities at low operating temperatures for solid-oxide fuel cells. Therefore, a co-doped technique was used to synthesize a highly ionically conductive two phase nanocomposite electrolyte Sr/Sm–ceria–carbonate by a co-precipitation method. A significant increase in conductivity was measured in this co-doped Sr/Sm–ceria–carbonate electrolyte at 550 °C as compared to the more commonly studied samarium doped ceria. The fuel cell power density was 900 mW cm−2 at low temperature (400–580 °C). The composite electrolyte was found to have homogenous morphology with a core–shell structure using SEM and TEM. The two phase core–shell structure was confirmed using XRD analysis. The crystallite size was found to be 30–60 nm and is in good agreement with the SEM analysis. The thermal analysis was determined with DSC. The enhancement in conductivity is due to two effects; co-doping of Sr in samarium doped ceria and it's composite with carbonate which is responsible for the core–shell structure. This co-doped approach with the second phase gives promise in addressing the challenge to lower the operating temperature of solid oxide fuel cells (SOFC).

 Please wait while we load your content...
Please wait while we load your content...