A comparative thermodynamic study of the formation of scandium complexes with DTPA and DOTA†
Abstract
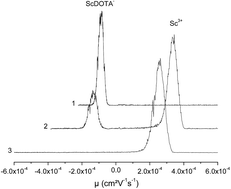
The complexation of scandium(III) by various polyaminopolycarboxylic ligands (DTPA and DOTA) was studied by capillary electrophoresis with ICP-MS detection in 0.1 mol L−1 NaCl ionic strength solutions at 25 °C. The results confirmed the formation of the 1 : 1 complexes for Sc(III)–DOTA and Sc(III)–DTPA systems. For each complex, the thermodynamic conditional constant was determined from the experimental data. The thermodynamic constants were extrapolated to zero ionic strength using the Davies equation and then compared to previously published data. These results were compared with free-ion selective radiotracer extraction (FISRE) data, which is a valid method for determining trace concentrations. The relative order of stability constants was preserved; as this method is experimentally simple, it is suitable for quick relative comparison of stability constant values under trace concentrations.

 Please wait while we load your content...
Please wait while we load your content...