In silico binding affinity to cyclooxygenase-II and green synthesis of benzylpyrazolyl coumarin derivatives†
Abstract
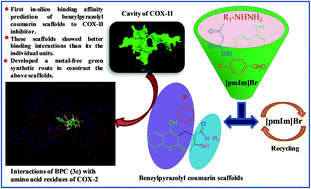
Here, we have reported for the first time, in silico binding affinity prediction of benzylpyrazolyl coumarin scaffolds to cyclooxygenase-II (COX-II) enzyme and a green synthetic route to access these scaffolds using a neutral ionic liquid, [pmIm]Br as the reusable catalyst and reaction media under metal-free conditions. The integrated scaffolds, benzylpyrazolyl coumarins exhibited stronger binding affinity to COX-II inhibitor than their individual moieties, 3-benzyl coumarins and pyrazolones. Moreover, 4-((4-hydroxy-2-oxo-2H-chromen-3-yl)(4-nitrophenyl)methyl)-5-methyl-2-phenyl-1H-pyrazol-3(2H)-one (3c) has shown even better binding affinity than the marketed anti-inflammatory drug, celecoxib.


 Please wait while we load your content...
Please wait while we load your content...