Enhancement of thermal and pH stability of an alkaline metalloprotease by nano-hydroxyapatite and its potential applications†
Abstract
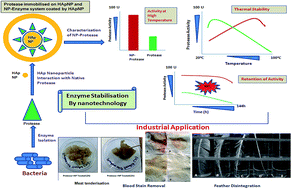
A purified calcium metalloprotease from Bacillus subtilis AKP(N) supplemented with hydroxyapatite nanoparticles [protease (+NP)] showed remarkable increase in thermostability (at 90 °C) over a broad alkaline pH range as compared to the non-supplemented enzyme. Thermodynamic analysis of the protease (+NP) showed decreases in Km and activation energy (Ea) with increased Vmax and deactivation energy (Ed) at both 40 °C and 90 °C. The protease (+NP) exhibited compatibility with various laboratory and commercial detergents (1%) at both 40 °C and 90 °C. The protease (+NP) was utilized to efficiently degrade feather to produce amino acids, removal of blood stains from cloth and tenderization of raw meat within a short time.

 Please wait while we load your content...
Please wait while we load your content...