Synthesis, spectroscopic characterization, X-ray analysis and theoretical studies on the spectral features (FT-IR, 1H-NMR), chemical reactivity, NBO analyses of 2-(4-fluorophenyl)-2-(4-fluorophenylamino)acetonitrile and its docking into IDO enzyme†‡
Abstract
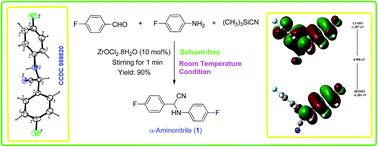
A new fluorinated α-aminonitrile compound, namely, 2-(4-fluorophenyl)-2-(4-fluorophenylamino)acetonitrile (C14H10F2N2), has been synthesized following a ‘green protocol’ and characterized on the basis of its elemental, detailed spectral and X-ray crystallographic analyses. The compound crystallizes in orthorhombic space group Pbca. The crystal structure has been solved by direct methods using single-crystal X-ray diffraction data obtained at room temperature and refined by full-matrix least-squares procedures to a final R-value of 0.0498 for 1430 observed reflections. The title compound has also been studied by means of theoretical calculations. The equilibrium geometry of α-aminonitrile has been obtained and analyzed using the DFT-B3LYP/6-311++G(d,p) method. Vibrational and NMR analyses have been performed by comparing calculated spectra with experimental observations. The reactivity of the title molecule is explained using various local as well as global molecular descriptors, and reactivity surfaces have also been analyzed. A molecular docking study of the title molecule in indoleamine 2,3-dioxygenase enzyme has also been carried out.

 Please wait while we load your content...
Please wait while we load your content...