Simultaneous adsorption of Cd(ii) and phosphate on Al13 pillared montmorillonite†
Abstract
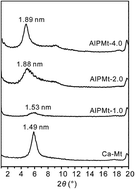
Al13 pillared montmorillonites (AlPMts) prepared with different Al/clay ratios were used to remove Cd(II) and phosphate from aqueous solution. The structure of AlPMts was characterized by X-ray diffraction (XRD), Thermogravimetric analysis (TG), and N2 adsorption–desorption. The basal spacing, intercalated amount of Al13 cations, and specific surface area of AlPMts increased with the increase of the Al/clay ratio. In the single adsorption system, with the increase of the Al/clay ratio, the adsorption of phosphate on AlPMts increased but that of Cd(II) decreased. Significantly enhanced adsorptions of Cd(II) and phosphate on AlPMts were observed in a simultaneous system. For both contaminants, the adsorption of one contaminant would increase with the increase of the initial concentration of the other one and increase in the Al/clay ratio. The enhancement of the adsorption of Cd(II) was much higher than that of phosphate on AlPMt. This suggests that the intercalated Al13 cations are the primary co-adsorption sites for phosphate and Cd(II). X-ray photoelectron spectroscopy (XPS) indicated comparable binding energy of P2p but a different binding energy of Cd3d in single and simultaneous systems. The adsorption and XPS results suggested that the formation of P-bridge ternary surface complexes was the possible adsorption mechanism for promoted uptake of Cd(II) and phosphate on AlPMt.


 Please wait while we load your content...
Please wait while we load your content...