Enhanced antibacterial activity of new “composite” biocides with both N-chloramine and quaternary ammonium moieties†
Abstract
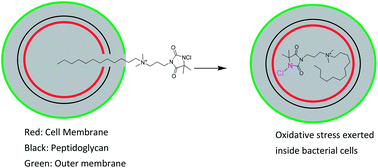
In view of the emerging resistance in bacteria against biocides, this work describes a novel combination of two existing biocides in one molecule to improve the bactericidal activity and overcome bacterial resistance. A new series of “composite” biocides combining an amide based N-chloramine with a quaternary ammonium (QA) moiety in one molecule was synthesized and the antibacterial kinetics of each biocide was tested against two clinically retrieved bacteria: methicillin-resistant S. aureus (MRSA) and multi-drug resistant (MDR) P. aeruginosa. The addition of multiple cationic centers into one N-chloramine molecule did not result in enhanced inactivation of bacteria. The bactericidal activity against both microbes increased dramatically when the length of the alkyl chain of QA moiety in these biocides increased to 12 and 14. Covalently bonding N-chloramine with long-chained QA moieties did result in faster kill of MRSA and MDR P. aeruginosa than the formulation with two separate counterparts (N-chloramine and long-chained QA salts). Uptake isotherm curves of the “composite” biocides with long alkyl chain substitution revealed more uptakes of the “composite” biocides by bacteria than the precursor mono-functional biocides (with only QA moieties). An improved antibacterial activity resulted from covalently bonding the two biocides (N-chloramine and long-chained QA salts) into one molecule.

 Please wait while we load your content...
Please wait while we load your content...