Anti-proliferative activities of flavone–estradiol Stille-coupling adducts and of indanone-based compounds obtained by SnCl4/Zn-catalysed McMurry cross-coupling reactions†
Abstract
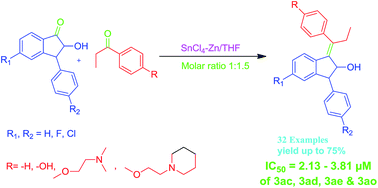
We described the synthesis of flavone–estradiol adducts and indanophen based tamoxifen analogs using a novel SnCl4–Zn reagent via a McMurry cross-coupling reaction and their anti-proliferative evaluation against human cervical cancer cell lines (HeLa) and human breast cancer cell lines (MCF-7 and MDA-MB-231). A library of 32 tamoxifen analogs was synthesized using indanone and propiophenone derivatives and evaluated for anti-proliferative activities. Among them, compounds 3ac, 3ad, 3ae and 3ao exhibited better anti-proliferative potencies (IC50 2.13–3.81 μM) than the drug doxorubicin (IC50 < 28 μM). The flavones–estradiol adducts 6ab and 6ad exhibited good anti-proliferative activity (IC50 2.85 ± 0.17 μM and 2.42 ± 0.23 μM; 3.64 ± 0.28 μM and 2.93 ± 0.14 μM) against breast cancer cells (MCF-7 and MDA-MB-231) respectively and IC50 2.17 ± 0.18 μM and 2.56 ± 0.32 μM against cervical cancer cells (HeLa) respectively than the standard drug. However, compounds 6ac, 6ae, 6af and 6ag showed moderate activity (IC50 < 10 μM). The structure–activity relationship analysis revealed that the optimal combination of side chains at the para-position of propiophenone and fluoro substituent on the indanone moiety enhanced the anti-proliferative activities of tamoxifen analogs.

 Please wait while we load your content...
Please wait while we load your content...