Copper-mediated aerobic oxidative synthesis of benzimidazo fused quinazolines via a multicomponent approach†
Abstract
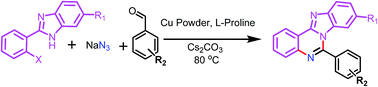
The first copper mediated aerobic oxidative multi-component synthesis of benzimidazo[1,2-c]quinazolines has been developed from 2-(2-halophenyl)benzoimidazoles, aldehydes and sodium azide as a nitrogen source. This protocol involves the formation of three C–N bonds starting from azidation of haloaryl with sodium azide followed by in situ conversion of azide into arylamine, which on condensation with benzaldehyde undergoes oxidative cyclization to afford benzimidazo[1,2-c]quinazoline in good to excellent yield.

 Please wait while we load your content...
Please wait while we load your content...