Rich synthetic and structural chemistry of polynuclear Pb(ii)–Cu(i)/Ag(i) heterobimetallic thiolate clusters, their decomposition and generation of a Cu(ii) hydrosulfide variant†
Abstract
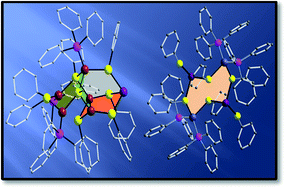
Three heterobimetallic clusters [{(PPh3)3}Cu5(μ-SPh)7Pb] (1), [Pb(μ-SPh)4{Cu(PPh3)2}2] (2), and [Pb2(μ-SPh)6{Ag(PPh3)2}2] (3) were synthesized and structurally characterized. The main skeleton of cluster 1 consists of five copper atoms and one lead atom arranged in an adamantane type stereochemistry, 2 is trinuclear with two copper atoms and one lead atom, while 3 adopts a cyclic 8-membered structure in a chair conformation with alternating silver and lead atoms bridged by thiophenolate sulfur atoms. The clusters 1 and 3 were subjected to solid state pyrolysis leading to the formation of lead sulfide. Solvent assisted decomposition of 1 yielded PbS nanoparticles along with an ionic copper(II) hydrosulfide complex [Cu(C6H5NCH3)4SH][PhSO3] (4). X-ray single crystal structure analysis for 1, reveals the formation of a dimer via lead–arene π-coordination with distances of 3.374 Å (lead–arenecentroid). Hirshfeld surface analyses and fingerprint plots quantify the interaction involved in 1. The nature of the interactions and the corresponding stabilization energies have been studied using DFT and AIM theory calculations.

 Please wait while we load your content...
Please wait while we load your content...