Piperazinylpyrimidine modified MCM-41 for the ecofriendly synthesis of benzothiazoles by the simple cleavage of disulfide in the presence of molecular O2†
Abstract
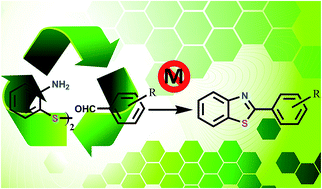
This is the first report of a sustainable and toxic metal free synthesis of benzothiazoles by the cleavage of ortho-aminothiophenol disulfide using a silica-supported heterogeneous catalyst in water in the presence of molecular O2 as a stoichiometric oxidant. For this purpose a new heterogeneous catalyst (MCM-PP) was synthesized by post synthesis grafting of 2-(piperazin-1-yl)pyrimidine functionalized organosilane onto MCM-41 mesoporous silica and it was characterized by BET surface area analysis, SAXRD, UHR TEM, CHN analysis, 13C CP MAS and 29Si MAS NMR. The reaction requires two different catalytic functions, i.e., an acidic one which is given by MCM-41 and a basic one, given by the organic base moiety anchored to the MCM-41. The greenness of the process was assured as water was exploited as the reaction medium and O2 from air as the stoichiometric oxidant. Furthermore the leaching of the active site can also be avoided as the organic moieties are covalently attached to the inorganic support. Standard leaching experiments proved that the reaction was heterogeneous with this recyclable catalyst.

 Please wait while we load your content...
Please wait while we load your content...