Unravelling the effect of flavonoids on the kinetic profiles of acrylamide in the Maillard reaction
Abstract
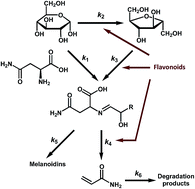
Acrylamide is mainly generated from asparagine and reducing sugars. The comprehensive kinetic profile of acrylamide and Maillard reaction products (MRP) is important for understanding the control of acrylamide. We unraveled the role of flavonoids in the kinetic process of acrylamide via a potato-based asparagine–glucose microwave heating system. Ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) was employed for the simultaneous determination of asparagine, glucose, fructose and acrylamide. The multi-response non-linear regression models were used for kinetic parameter estimation. The results indicated a positive role of flavonoids in the fructose-participating (k3: 0.461–0.674; P < 0.05, P < 0.01, compared to the control) instead of the glucose-participating Maillard reaction (k1: 0.128–0.148; P > 0.05). The addition of flavonoids significantly reduces the formation of acrylamide during the advanced stage (k4: 2.311–4.327; P < 0.05, P < 0.01) but did not affect its elimination process (k6: 0.076–0.113; P > 0.05). Treatment of flavonoids has no significant impact on the formation of melanoidins (k5: 3.645–4.368; P > 0.05) and retains the original sensory attributes of MRP. These findings favor thinking mitigation strategies via the use of additives during food processing.

 Please wait while we load your content...
Please wait while we load your content...