A direct approach for the expedient synthesis of unsymmetrical ethers by employing bromodimethylsulfonium bromide (BDMS) mediated C–S bond cleavage of naphthalene-2-ol sulfides†
Abstract
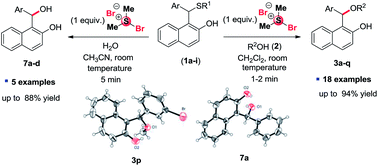
The unsymmetrical ether derivatives 1-(alkoxy(aryl)methyl)naphthalen-2-ols (3a–q) were synthesized from 1-(aryl(alkyl/arylthio)methyl)-naphthalene-2-ol derivatives (1) and alcohols (2) by cleavage of C–S bond using bromodimethylsulfonium bromide (BDMS) at room temperature. Moreover, 1-(hydroxy(aryl)methyl)naphthalen-2-ol derivatives (7a–d) were also synthesized from the corresponding sulfide derivatives (1) on reaction with water using one equivalent BDMS. The direct synthesis of 3a was also achieved from β-napthol 5 by tuning the reaction conditions with overall one-pot two-steps sequence. Interestingly, the desired products 3a are not accessible directly from 2-naphthol, aromatic aldehyde and alcohol in the presence of BDMS at room temperature. Some of the salient features of this protocol are mild reaction conditions, good yields, operational simplicity and with a wide substrate scope.

 Please wait while we load your content...
Please wait while we load your content...