Synthesis and controlled curcumin supramolecular complex release from pH-sensitive modified gum-arabic-based hydrogels†
Abstract
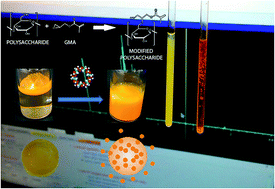
Curcumin (CUR) is a polyphenolic compound including a beta-diketone moiety, which is associated with numerous pharmacological activities, but applications are limited due to its low water solubility. Thus, in this work some inclusion complexes of CUR with alpha-cyclodextrin (α-CD) and beta-cyclodextrin (β-CD) were prepared using different host : guest proportions to improve drug solubilization in biological fluids. The formation of these complexes was confirmed by 1H NMR and thermogravimetric analysis. The stoichiometries of the CUR/α-CD and CUR/β-CD complexes were 1 : 1 and 1 : 2 and the association constants were 344 mol−1 L and 7.2 × 107 mol−2 L2 for α-CD and β-CD, respectively. The major stability of the CUR/β-CD complex is justified by an inclusion of the aromatic ring inside the CD cavity, whilst in the case of α-CD-complexes the interactions occur via H-bridges, showing the latter complexes’ slow exchange on the NMR time-scale. Even so, the solubility of curcumin complexes is clearly controlled by the solubility of CDs, showing the highest solubility for CUR/α-CD complexes. Hydrogels of modified gum arabic containing CUR/α-CD (1 : 4) were obtained and used for controlled release of CUR in simulated intestinal fluid (SIF) and simulated gastric fluid (SGF). The kinetics of release was pH-responsive and the percentage of CUR released was ca. 97% in SIF and 6.7% in SGF. For the toxicity studies on undifferentiated Caco-2 cells, IC50s of 63.4 ± 14.4 μg mL−1 and 85.2 ± 14.9 μg mL−1 for CUR and CUR/α-CD (1 : 4), respectively, were obtained. The toxicity of these samples on differentiated Caco-2 cells was lower than on undifferentiated cells. Additionally, the CUR incorporated into hydrogels showed no toxic effects on differentiated and undifferentiated Caco-2 cells, indicating the pharmaceutical potential of three-dimensional matrices of GAm for controlled release of CUR complexed with cyclodextrin.

 Please wait while we load your content...
Please wait while we load your content...