Endophyte inspired chemical diversity from beta-caryophyllene†
Abstract
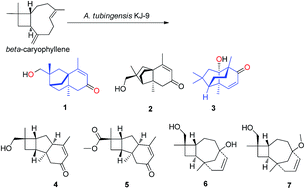
The natural product (−)-β-caryophyllene is considered as an ideal initiator to generate diverse scaffolds by transannular cyclization due to its macrocyclic structure and abundant availability in nature. An endophytic strain Aspergillus tubingensis KJ-9 was screened in our lab to catalyse remodeling of the β-caryophyllene skeleton. In the fermentation, macrocyclic β-caryophyllene was remodeled into a series of natural product-like polycyclic compounds with unusual diverse skeletons (1–7). Their structures, including absolute configuration, were elucidated by several spectroscopic methods (1D & 2D NMR, HRMS, ECD and X-ray diffraction). Especially, scaffolds 1 and 3 were not found in acid-catalysed products before. Our findings demonstrated the potential of endophytic fungi for catalyzing macrocyclic compounds into diverse products.

 Please wait while we load your content...
Please wait while we load your content...