Hydrothermal properties of the COS/D2 water model: a polarizable charge-on-spring water model, at elevated temperatures and pressures†
Abstract
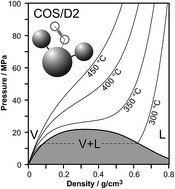
Molecular simulations have been conducted to assess the pVT properties and static permittivity of the charge-on-spring polarizable water model COS/D2 at hydrothermal conditions from 300 to 450 °C and bulk densities of 0.001 to 1.0 g cm−3. The results indicate that the model performs well for reproducing volumetric and dielectric properties of real water. The liquid–vapor coexistence curve and critical point of COS/D2 water (ca. 334 °C, 21.7 MPa and 0.32 g cm−3) are also in reasonable agreement with those of real water (ca. 374 °C, 22.1 MPa and 0.322 g cm−3). We include comparisons to other polarizable (as well as non-polarizable) water models where available. The good performance of the COS/D2 model at high temperatures and pressures is noteworthy considering that the model was parameterized exclusively at room temperature and pressure. Moreover, these results imply that COS/D2 is a suitable water model for simulating solutions of non-electrolyte and electrolyte solutes at elevated temperatures and pressures.

 Please wait while we load your content...
Please wait while we load your content...