Lipase-catalyzed regioselective domino reaction for the synthesis of chromenone derivatives†
Abstract
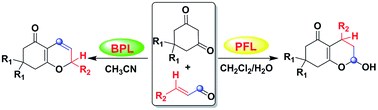
An efficient synthesis of 2H-chromenones and 2-hydroxyl-2H-chromenones derivatives has been developed from 1,3-dicarbonyls and α,β-unsaturated aldehydes by a controllable regioselective domino cyclization reaction under catalysis of different lipases. 2H-Chromenones derivatives were synthesized by bovine pancreatic lipase (BPL) in acetonitrile, while lipase from Pseudomonas fluorescens (PFL) can catalyze the synthesis of the 2-hydroxyl-2H-chromenones derivatives in dichloromethane with moderate to high yields.

 Please wait while we load your content...
Please wait while we load your content...