Photoassisted hetero-Fenton degradation mechanism of Acid Blue 74 by a γ-Fe2O3 catalyst†
Abstract
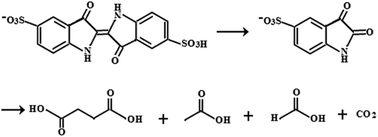
Fusiform-like maghemite particles were prepared through a distinct two-step transformation from Fe(II) to γ-FeOOH and then to γ-Fe2O3 by precipitation and calcination respectively. The structure and property of the as-prepared sample were characterized by XRD, SEM, XPS, UV-vis and FTIR. The degradation of Acid Blue 74 by γ-Fe2O3 was investigated under visible light irradiation. 1H NMR, TOC, UV-vis, direct infusion-ESI-(−)MS and FTIR spectroscopic techniques provide an insight into the nature of the degradation products. A more complete degradation mechanism of Acid Blue 74 on γ-Fe2O3 was presented. The results indicate the total degradation rate is close to 100% for a 100 mg L−1 Acid Blue 74 solution. Among them, about 60% was completely mineralized and the other 40% was degraded to aliphatic acids. The magnetic γ-Fe2O3 could be conveniently recovered by applying a magnet and recycled. The γ-Fe2O3 retained 100% degradation capacity for Acid Blue 74 after 6 cycles.

 Please wait while we load your content...
Please wait while we load your content...