Design, synthesis and biological evaluation of novel podophyllotoxin derivatives bearing 4β-disulfide/trisulfide bond as cytotoxic agents†
Abstract
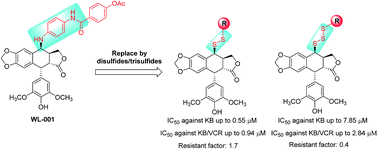
A novel series of C-4β-disulfide/trisulfide-containing podophyllotoxin derivatives were designed, synthesized, and biologically evaluated for their cytotoxic activities against human cancer cell lines, including KB (Mouth Epidermal Carcinoma Cells) and KB/VCR (Vincristine-resistant Mouth Epidermal Carcinoma Cells). Most of these compounds exhibited promising moderate to good cytotoxic activities. In particular, some of them displayed even superior activities to that of etoposide, especially for KB/VCR cell lines, indicating that introduction of the disulfide/trisulfide moiety would be beneficial for overcoming the multi-drug resistant limitation of etoposide. Moreover, the metabolic evaluation of the most promising compound was further performed to reveal that disulfide bond can be stable in human plasma over 8 hours, indicating good potential of these compounds for in vivo anti-cancer activities.

 Please wait while we load your content...
Please wait while we load your content...