The origin for highly enantioselective induction of 1-naphthol to isatin-derived N-Boc ketimines catalyzed by quinine thiourea catalyst: an experimental and computational study†
Abstract
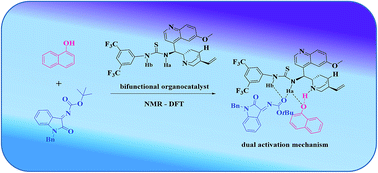
An enantioselective aza-Friedel–Crafts reaction of 1-naphthol with isatin derived N-Boc ketimines by cinchona based bifunctional thiourea as organo-catalyst is reported. In general the derivatives of Betti base are formed in excellent enantioselectivities (95–99%) with high yields (<99%). The combination of experimental and computational studies have revealed the origin of the stereoselectivity of the aza-Friedel–Crafts reaction of 1-naphthol with isatin derived N-Boc ketimines by cinchona based bifunctional thiourea as organo-catalyst. The attractive electrostatic interactions in the Re face transition state and the deleterious lone-pair⋯π interactions in the Si face transition state governed the formation of Re-face as a major product in this aza Friedel–Crafts reaction. The NMR studies performed to examine the formation of the complex with 1-naphthol, isatin derived N-Boc ketimines and cinchona based bifunctional thiourea catalyst has corroborated the calculated complex geometry employed in the study.

 Please wait while we load your content...
Please wait while we load your content...