Synthesis of benzochromenes and dihydrophenanthridines with helical motifs using Garratt–Braverman and Buchwald–Hartwig reactions†
Abstract
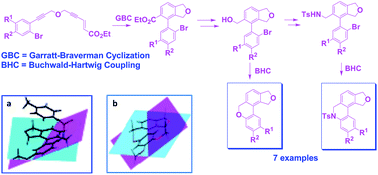
A simple strategy for the synthesis of 6H-benzo[c]chromenes and 5,6-dihydrophenanthridines through a judicious use of Garratt–Braverman (GB) cyclization and Buchwald–Hartwig (BH) coupling in moderate to good yield, has been reported. The uniqueness of the GB reaction is exemplified in providing the required biaryl intermediate which could be successfully converted to the target skeleton via functional group transformations followed by BH coupling. The presence of a dihydro-isofuran moiety assisted in inducing a helical motif in these molecules, which is also confirmed by single crystal X-ray structural analysis. The crystallographic data gives valuable insight into the role of the non-bonding interaction in regulating the helicity.

 Please wait while we load your content...
Please wait while we load your content...