Evaluation of toxicity and biodegradability for cholinium-based deep eutectic solvents†
Abstract
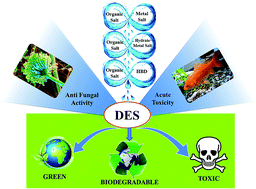
This study presented the toxicological and biodegradable assessment of different cholinium-based deep eutectic solvents (DESs). They were formed from choline chloride (ChCl) and N,N-diethyl ethanol ammonium chloride (EAC) as salts and four hydrogen bond donors, namely ethylene glycol (EG), glycerol (Gly), urea (U), malonic acid (MA), in addition to a metal salt, i.e. zinc chloride (ZnCl2), and a hydrated metal salt, i.e. zinc nitrate hexahydrate (ZnN). The toxicity towards Aspergillus niger of pure and aqueous DESs was evaluated by observing the inhibition zone using an agar well diffusion assay, and the minimum inhibition concentration (MIC) using a broth dilution assay, respectively. The MIC values of the DESs varied from 1 to 650 mg mL−1, whereas the inhibition zones changed according to the DES dose amount. Another test for acute toxicity was performed by evaluating the lethal concentration at 50% (LC50) of the same DESs on Cyprinus carpio fish. The LC50 of DESs ranged from practically harmless (e.g. ChCl : EG-DESaq) to highly toxic (e.g. EAC : ZnCl2-DESaq). The toxicity profile of the DESs depended on their concentration, type of individual components, and interaction with living organisms. Moreover, the DESs recorded higher toxicity compared to their individual components on fungi. However, lower toxicity was found for the DESs tested on Cyprinus carpio. Types I (organic salts and metal salt) and II (organic salt and hydrate metal salt) eutectics exhibited significantly higher toxicity than type III (organic salts and HBD). This was due to the presence of the innate toxicity of the metal salts. The biodegradability was appraised by a closed bottle test in which all the DESs were found to be readily biodegradable. To the best of our knowledge, there are no previous studies reported regarding the toxicity of cholinium-based DESs on freshwater fish or fungi and the biodegradability of EAC-based DESs. Therefore, this investigation can be used as a benchmark for future development of DESs.

 Please wait while we load your content...
Please wait while we load your content...