Synthesis and electronic properties of N-heterocyclic carbene-containing conducting polymers with coinage metals†
Abstract
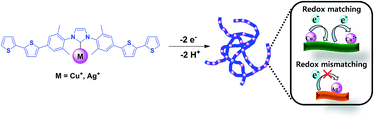
Following the recent development of functional materials based on metal-containing conducting polymers, we designed and synthesized a series of bithiophene based N-heterocyclic carbene (NHC) conducting polymers containing coinage metals (Cu, Ag, and Au). Absorption spectroscopy, NMR spectroscopy, cyclic voltammetry, and X-ray photoelectron spectroscopy (XPS) were used to analyze the monomers and the polymer thin films. The peak currents of the electrodeposited films on Pt-button electrodes increased proportionally with the scan rate, exhibiting the stability of the polymer thin films. Remarkably, in the electrochemical studies of the coinage metal complexes, the redox potential of Cu+/Cu2+ perfectly matched with the redox wave of quaterthiophene moieties of the polymer backbone. However, the redox potential of Ag/Ag+ did not match with the redox potential of the polymer backbone. During the oxidation process in the spectroelectrochemical studies, the conducting coinage metallopolymers exhibited a drastic color change from orange (reduced form) to dark green (oxidized form), and they can be envisioned in the applications of electrochromic materials.

 Please wait while we load your content...
Please wait while we load your content...