Highly efficient biotransformation of ginsenoside Rb1 and Rg3 using β-galactosidase from Aspergillus sp.
Abstract
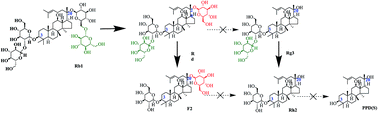
A preliminary study on the enzymatic biotransformation of ginsenosides is evaluated. β-Galactosidase from Aspergillus sp. displayed β-glucosidase activity, which was responsible for its ability to transform major ginsenoside Rb1 to rare ginsenoside F2 via ginsenoside Rd. The Rb1 conversion, Rd and F2 yields reached 100%, 80.7% and 14.3% after 60 h at 60 °C, respectively. Ginsenoside Rg3 can be selectively hydrolyzed and only Rh2 was obtained with this β-galactosidase as well. Before hydrolysis, an Rg3 inclusion complex was prepared with hydroxypropyl-β-cyclodextrin (HP-β-CD) to improve the aqueous solubility. The solubility of Rg3 increased 74.6 fold, and the phase solubility curve displayed a typical AL-type, which indicates the formation of a 1 : 1 inclusion complex. Using an enzyme loading of 500 U g−1 Rg3, the highest Rg3 conversion of 90.6% and Rh2 yield of 88.5% were obtained after 24 h at 60 °C. These results indicate that β-galactosidase from Aspergillus sp. could be useful for the mass production of rare ginsenosides.

 Please wait while we load your content...
Please wait while we load your content...