Direct access to functionalized 4-nitromethyl-chromenes via a domino reaction under catalyst-free conditions†
Abstract
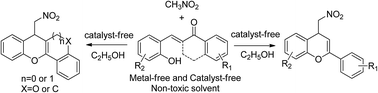
A catalyst-free tandem reaction for synthesis of 4-nitromethyl-chromenes has been established from accessible α,β-unsaturated ketones and CH3NO2. Target products were obtained in non-toxic solvent under catalyst-free conditions. Especially, the scope of substrates was expanded to tricyclic α,β-unsaturated ketones for the synthesis of tetracyclic heterocompounds in medium to good yields.

 Please wait while we load your content...
Please wait while we load your content...