Application of aziridinium ring opening for preparation of optically active diamine and triamine analogues: highly efficient synthesis and evaluation of DTPA-based MRI contrast enhancement agents†
Abstract
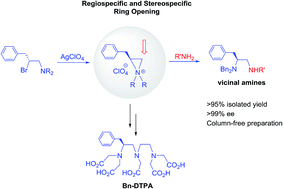
Ring opening of aziridinium ions with nitrogen nucleophiles was applied to the highly efficient synthesis of optically active vicinal diamines and diethylene triamine pentaacetic acid (DTPA) analogues as potential magnetic resonance imaging (MRI) contrast enhancement agents. The synthetic method features a column-free isolation of the regiospecific and stereospecific nucleophilic substitution products of enantiomerically enriched aziridinium ions in excellent yield.

 Please wait while we load your content...
Please wait while we load your content...