Metal-free regioselective C-3 acetoxylation of N-substituted indoles: crucial impact of nitrogen-substituent†
Abstract
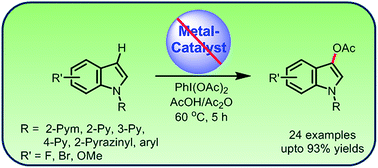
A metal-free method for the regioselective C-3 acetoxylation of the N-substituted indoles with PhI(OAc)2 is described under mild reaction conditions. This method tolerates a broad range of functional groups with moderate to good yields. The π-electron-deficient aryl-substituents on the N-atom of indoles and the acidic reaction medium remarkably favor C-3 acetoxylation.

 Please wait while we load your content...
Please wait while we load your content...