Palladium-catalyzed ligand-free and efficient Suzuki–Miyaura reaction of heteroaryl halides with MIDA boronates in water†
Abstract
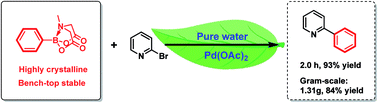
A simple and environment-friendly protocol for the palladium-catalyzed ligand-free Suzuki–Miyaura reaction of heteroaryl halides with N-methyliminodiacetic acid (MIDA) boronates is developed. The reaction is performed in water as the sole medium and allows the preparation of a variety of heterobiaryls in excellent yields.

 Please wait while we load your content...
Please wait while we load your content...