Photophysical and theoretical studies of peripherally halogenated octaphenoxyphthalocyanines†
Abstract
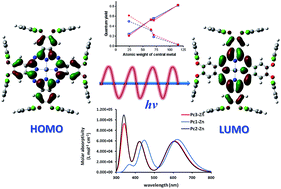
Peripherally substituted phthalocyanines with halogenated phenoxy groups containing different central metals (In(III), Ga(III), Mg(II) and Zn(II)) were synthesized. X-ray analyses confirmed that the halogen atoms (Cl, Br) at the ortho positions were bulky enough to orient the phenoxy substituents perpendicularly relative to the Pc core and perfectly discouraged cofacial aggregation. The ground state geometry, HOMO–LUMO energies and the electronic properties were theoretically calculated for some complexes and corresponded well with experimental data. Analysis of fluorescence and singlet oxygen quantum yields revealed a strong heavy atom effect provided by the central metal. Irrespective of the present halogen, indium(III) complexes were characterized by high singlet oxygen production and very low fluorescence while reverse data were obtained for Mg(II) complexes.

 Please wait while we load your content...
Please wait while we load your content...