Hydrothermal synthesis of bimodal mesoporous MoS2 nanosheets and their hydrodeoxygenation properties†
Abstract
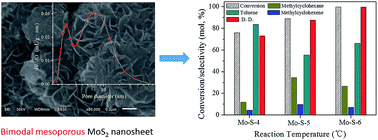
In this study, bimodal mesoporous MoS2 nanosheets were successfully synthesized by a hydrothermal method. The effect of pH value, pressure, time and temperature in the preparation process of MoS2 on its structure property and catalytic activity were studied in detail. Low pH value and pressure were beneficial for the preparation of a MoS2 nanosheet with a large surface area and narrow bimodal pore distribution, which exposed more effective active sites on the surface and provided suitable space for reactants and products to diffuse in less resistance. But the acceleration hydrolysis of CS(NH2)2 at the low pH value enhanced the formation rate of MoS2 and then weakened the nanosheet structure. In the HDO of p-cresol, MoS2 exhibited high catalytic activity, and the dominant route was direct deoxygenation. After 4 h, both the conversion and deoxygenation degree reached 99.9% at 300 °C, and toluene selectivity was 66.2%. The HDO reaction mechanism could be well explained by the Rim-Edge model. The higher conversion in the HDO of p-cresol on MoS2 depended on the larger surface area and greater number of big pores of the catalyst, while the higher direct deoxygenation activity of MoS2 depended on the greater number of layers in its stacks.

 Please wait while we load your content...
Please wait while we load your content...