Importance of polarization assisted/resonance assisted hydrogen bonding interactions and unconventional interactions in crystal formations of five new complexes bearing chelidamic acid through a proton transfer mechanism†
Abstract
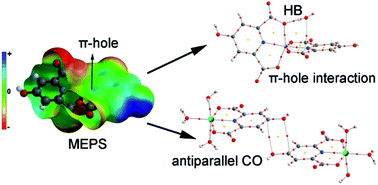
(H4a-dmpy)[Cr(Hcda)2]·4H2O (1), (H2a-4,6-dmpym)[Cr(Hcda)2]·3H2O·2a-4,6-dmpym (2), [M(Hcda)(H2O)3]·H2O (3) and (4) (M = CoII and NiII, respectively) and (H2a-4mpy)2[FeCl3(Hcda)]·3H2O (5) (H3cda = 4-hydroxypyridine-2,6-dicarboxylate or chelidamic acid; 4a-dmpy = 4-aminodimethylpyridine; 2a-4,6-dmpym = 2-amino-4,6-dimethylpyrimidine; 2a-4mpy = 2-amino-4-methylpyridine) are five new coordination compounds obtained through the same proton transfer mechanism. The chemical formulas and structures for compounds 1, 2 and 5 are determined by means of single crystal X-ray diffraction, elemental analysis and IR spectroscopy. Single crystal X-ray diffraction was used to characterize compounds 3 and 4. From these results, it has been found that 1, 2 and 5 are ionic with protonated amines as cations and chelidamic acid-complexed metals as anions while 3 and 4 are neutral chelidamic acid complexes of CoII and NiII with the same 3D networks in the crystal. The geometries of all these complexes with Hcda are distorted octahedral around the metal center. Chelidamic acid, as a tridentate ligand, adopts its most common coordination mode through one oxygen atom of each carboxylate group and a nitrogen atom of its pyridine ring in all five complexes. Among various types of non-covalent interactions which result in the stabilized MOFs, hydrogen bonds, particularly very strong polarization assisted hydrogen bonding (PAHB) as well as resonance assisted hydrogen bonding (RAHB), appear to dominate. We have also studied other unconventional interactions observed in the solid state of the complexes using high level DFT calculations. Solution studies were performed to fully characterize the new compounds.

 Please wait while we load your content...
Please wait while we load your content...