Role of lithium oxide as a sintering aid for a CGO electrolyte fabricated via a phase inversion technique
Abstract
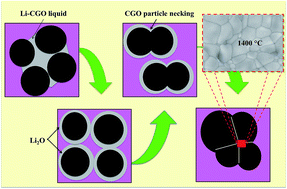
The incorporation of lithium oxide (Li2O) as a sintering additive has specific advantages for electrolyte membrane fabrication. However, the viability of the sintering additive to be implemented in a phase inversion technique is still ambiguous. In this first attempt, lithium was doped into a gadolinium-doped ceria (CGO) crystal structure using the metal nitrate doping method and calcined at four different temperatures, i.e. 140, 300, 500 and 700 °C. The prepared Li-doped CGO (Li–CGO) powders were analyzed by thermal gravimetric analysis (TGA), differential scanning calorimetry (DSC), X-ray diffraction (XRD), N2 adsorption/desorption, and Fourier-transform infrared (FTIR). Primary results demonstrate that the calcination temperature of the Li–CGO influences the condition of the electrolyte suspension. Li–CGO calcined at 700 °C (D-700), as compared with other Li–CGO, possessed a strong interaction between the Li and CGO. The D-700 was then incorporated into the electrolyte flat sheet membrane which was prepared by a phase inversion technique. The membrane was then sintered at different sintering temperatures from 1350 °C to 1450 °C. In comparison with the unmodified CGO, the morphological results suggest that the Li2O can remarkably promote the densification of CGO at a lower sintering temperature (1400 °C). These findings help to promote the use of sintering additives in a ceria-based electrolyte suspension specifically for the phase inversion technique.

 Please wait while we load your content...
Please wait while we load your content...