Homogeneous benzoylation of cellulose in 1-allyl-3-methylimidazolium chloride: Hammett correlation, mechanism and regioselectivity
Abstract
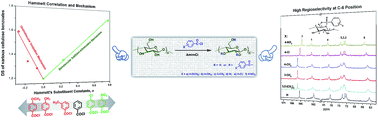
Homogeneous benzoylation of cellulose with a series of substituted benzoyl chlorides, in which substituents varied from electron donating to electron withdrawing groups, was investigated in an ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl). The electronic effect of substituents had a considerable effect on the reaction. A plot of Hammett parameters of substituents vs. degree of substitution (DS) of resultant cellulose esters exhibited a V-shaped graph: a negative slope for negative Hammett parameters, and a positive slope for positive Hammett parameters. Such a Hammett plot indicated the mechanism of cellulose benzoylation: the reaction underwent a unimolecular ionization mechanism with a carbocationic intermediate when benzoyl chlorides were with electron-donating substituents, and a bimolecular addition–elimination mechanism with a tetrahedral intermediate when benzoyl chlorides were with electron-withdrawing substituents. In addition, 13C-NMR analysis showed that most of the substituted benzoyl chlorides exclusively preferred the primary hydroxyl at the C-6 position. This unusually high regioselectivity was attributed to the synergistic effect of appropriate reaction rate, moderate steric effect and reaction mechanism of benzoylation.

 Please wait while we load your content...
Please wait while we load your content...