Synthesis, characterization and targeted cell imaging applications of poly(p-phenylene)s with amino and poly(ethylene glycol) substituents†
Abstract
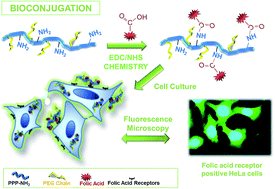
A novel approach for bioconjugation associated with a fluorescent conjugated polymer is demonstrated. For this purpose, a conjugated polymer, poly(p-phenylene) (PPP), with lateral substituents, namely primary amino groups and poly(ethylene glycol) (PEG) chains, as a potential building block for polymer bioconjugates was synthesized and characterized. The synthesis was achieved through Suzuki polycondensation reaction in the presence of Pd(PPh3)4 catalyst by using independently prepared PEG and amino functionalized dibromo benzenes in conjunction with benzene diboronic acid. For the evaluation of the bioactive PPP labeled with folic acid (FA) as a potential targeted cell imaging probe, HeLa and A549 cancer cells were used. Cytotoxicity assay showed that the polymer was not toxic to either of the cells. Additionally, the fluorescence images showed that, depending on the level of the FA receptors on the cell surfaces, the fluorescent intensity in HeLa cells was obviously higher than A549 cells when treated with FA conjugated PPP-NH2-g-PEG polymer. The resulting FA/PPP-NH2-g-PEG conjugate was successfully used as a bioconjugate for targeting and specifically imaging FA receptor positive HeLa human cervical cancer cells.

 Please wait while we load your content...
Please wait while we load your content...