Nanodiamond–TiO2 composites for photocatalytic degradation of microcystin-LA in aqueous solutions under simulated solar light
Abstract
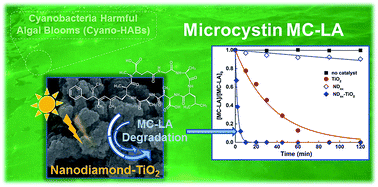
Titanium dioxide (TiO2) has been under intensive investigation for photocatalytic degradation of cyanobacterial toxins. In order to develop more efficient photocatalysts, TiO2 and oxidized nanodiamonds (NDox) were combined as a composite catalyst (NDox–TiO2), which was tested in the photocatalytic oxidation of microcystin-LA (MC-LA), a cyanotoxin frequently found in freshwater. NDox–TiO2 and neat TiO2 photocatalysts were prepared by a liquid phase deposition method. A wide variety of analytical techniques, including physical adsorption of nitrogen, X-ray diffraction (XRD), UV-Vis and IR diffuse reflectance spectroscopies (DRUV-Vis and DRIFT), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM), were used to characterize the materials. The performance of the photocatalysts was studied under both simulated solar and visible light. The kinetic results show remarkable efficiency for the NDox–TiO2 composite under simulated solar light irradiation with a synergistic factor of more than 15 relative to neat TiO2, while negligible photocatalytic activity was observed for the degradation of MC-LA when NDox–TiO2 was used under visible light illumination due to the wide band gap of the composite material. The photocatalytic efficiency of NDox–TiO2 was ascribed to the good dispersion of both phases in the composite material, facilitating the possible electronic interaction at the heterojunction interface between NDox and TiO2.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners

 Please wait while we load your content...
Please wait while we load your content...