A rapid metal free synthesis of 5-substituted-1H-tetrazoles using cuttlebone as a natural high effective and low cost heterogeneous catalyst†
Abstract
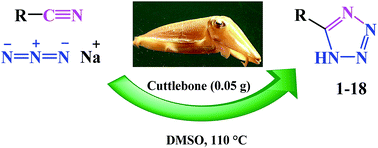
A convenient, rapid and metal free synthesis of 5-substituted-1H-tetrazoles is described by [3 + 2] cycloaddition reaction of nitriles with sodium azide. The reaction was catalyzed by cuttlebone in DMSO at 110 °C. Cycloaddition reaction of nitriles with sodium azide happened in the presence of mesoporous cuttlebone by “electrophilic activation” of nitriles through hydrogen bond formation between the cuttlebone and nitrile. Cuttlebone as a natural low cost heterogeneous catalyst with high porosity, high flexural stiffness, high compressive strength and high thermal stability affords 5-substituted-1H-tetrazoles rapidly with high efficiency.
- This article is part of the themed collection: Organic chemist’s toolbox

 Please wait while we load your content...
Please wait while we load your content...