Competition between molecular and dissociative adsorption of hydrogen on palladium clusters deposited on defective graphene
Abstract
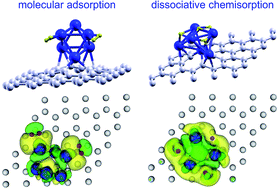
The contribution of Pd doping to enhance the hydrogen storage capacity of porous carbon materials is investigated. Using the Density Functional Formalism, we studied the competition between the molecular adsorption and the dissociative chemisorption of H2 on Pd clusters anchored on graphene vacancies. The molecular adsorption of H2 takes place with energies in the range of 0.7–0.3 eV for adsorption of one to six hydrogen molecules. Six molecules saturate the cluster, and additional hydrogen could only be adsorbed, with much smaller adsorption energies, at farther distances from the cluster. Dissociative chemisorption is the preferred adsorption channel from one to three hydrogen molecules, with adsorption energies in the range of 1.2–0.6 eV. After the first three molecules are dissociatively chemisorbed, three additional hydrogen molecules can be adsorbed non-dissociatively onto the Pd cluster with adsorption energies of 0.5 eV. The desorption of Pd–H complexes is prevented in all cases because the Pd clusters are firmly anchored to graphene vacancies. Our results are very promising and show that Pd clusters anchored on graphene vacancies retain their capacity to adsorb hydrogen and completely prevent the desorption of Pd–H complexes that would spoil the hydrogen releasing step of the cycle.

 Please wait while we load your content...
Please wait while we load your content...