Microscopic study of the equation of state of β-HMX using reactive molecular dynamics simulations
Abstract
The equation of state (EoS) is the relation between physical quantities describing thermodynamic states of materials under a given set of conditions such as pressure, temperature, and volume. The EoS plays a significant role in determining the characteristics of energetic materials, including Chapman–Jouguet point and detonation velocity. Furthermore, the EoS is the key to connect microscopic and macroscopic phenomena in the study of energetic materials. For instance, the foundation of the ignition and growth model for high explosives is two Jones–Wilkins–Lee (JWL) EoSs, one for unreacted explosive and the other for reacted explosive. Thus, an accurate calculation of the EoS is required for the study of energetic materials. In this paper, the EoSs for both unreacted and reacted β-HMX are calculated using molecular dynamics simulations with the ReaxFF-lg potential. The microscopic simulation results are compared with experiments, first-principles calculations, and a continuum ignition and growth model. Good agreement is observed. Our result indicates that molecular dynamics simulations are a reliable alternative method to experiments to generate the EoS for energetic materials.
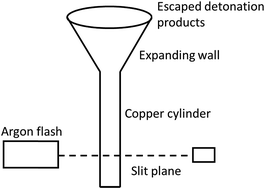

 Please wait while we load your content...
Please wait while we load your content...