I2/Cu-mediated self-sorting domino reaction of aryl β-ketoesters into symmetrical 2-carboalkoxy-1,4-enediones: application to synthesis of pyrazine, β-carboline and quinoxalines†
Abstract
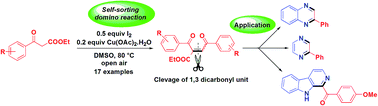
A self-sorting domino reaction of aryl β-ketoesters into symmetrical 1,4-enediones is reported by an I2/Cu system. The reaction proceeds through tandem iodination, self-dimerization and Krapcho dealkoxycarbonylation in one pot under open air condition. Further, 1,4-enediones were successfully employed for the synthesis of bioactive pyrazine, β-carboline and quinoxalines via aza-Michael addition, intramolecular cyclization and C–C bond cleavage of 1,3-dicarbonyl unit under mild reaction condition.

 Please wait while we load your content...
Please wait while we load your content...