Synthesis and characterization of novel symmetrical two-photon chromophores derived from bis(triphenylaminotetrathienoacenyl) and fused-thiophene units†
Abstract
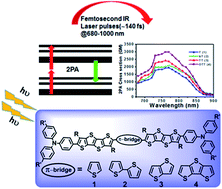
Four new donor–π–donor (D–π1–π2–π1–D) fused-thiophene-based chromophores, end-functionalized with electron-donating triphenylamine (TPA) groups, were developed and characterized for a two-photon absorption study. Within this series, tetrathienoacene (thieno[2′,3′:4,5]thieno[3,2-b]thieno[2,3-d]thiophene; TTA) moieties were employed as side-conjugated (π1) units, and the central conjugated core (π2) units were altered with thiophene (T), bithiophene (bT), thienothiophene (TT), and dithienothiophene (DTT) for chromophores 1–4, respectively. The structural and photophysical relationships of the four compounds were compared, and all four chromophores showed strong fluorescence with good thermal stability. The energy gap compression of these chromophores was verified by electrochemistry and density functional theory (DFT) calculations. The two-photon-related properties of 1–4 were examined using femtosecond laser pulses as the probing tool. The magnitude of the two-photon absorptivity was found to be strongly dependent on the molecular conjugation length and the center fused-thiophene unit. Within the family, the most conjugated DTT-centered chromophore (4) exhibits the strongest and the most widely dispersed two-photon absorption cross-section value up to 3000 GM. To the best of our knowledge, this is the highest 2PA cross section value reported to date among the studied fused thiophene-based chromophores.

 Please wait while we load your content...
Please wait while we load your content...