Grafting Ni particles onto SBA-15, and their enhanced performance for CO2 methanation†
Abstract
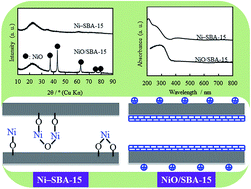
By a post synthesis method, nickel (Ni) particles could be grafted onto SBA-15 for the first time through chemical bond (–O–Ni–O–Si–O–) formation between silicon (Si) and Ni via oxygen (O) using Ni ammonia (NH3) complex ions (Ni(NH3)x)2+ with an NH3/Ni mole ratio of 1–5, which existed as Ni phyllosilicate on the SBA-15 surface, while Ni particles could not be grafted onto SBA-15 in the absence of NH4OH (NH3/Ni mole ratio of 0). An NH3/Ni mole ratio of 2–4 was suitable for grafting conditions, which could give a product with the closest Ni amount to that of raw Ni complex ion solution. The product obtained was named as the Ni-grafted SBA-15 sample. XPS, UV-vis and H2-TPR analyses demonstrated that a chemical bond was formed between Ni and silicon (Si) via oxygen (O), and no bulk nickel oxides existed in the Ni-grafted SBA-15 sample. The formation of –O–Ni–O–Si–O– was completed via the reaction between hydrolyzate (Ni(OH)(NH3)x−1)+ from (Ni(NH3)x)2+ and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–OH (silanol sites) on the SBA-15 surface. The Ni-grafted SBA-15 catalyst suited CO2 methanation, resulting in higher CO2 conversion and methane selectivity than a NiO dispersed SBA-15 catalyst obtained by the conventional post synthesis method. The activation energy for CO2 methanation increased with a decreasing initial Ni amount used. The rate equation for CO2 methanation could be expressed as: r = kCCO20.64CH24.05, where C is the concentration. The Ni-grafted SBA-15 catalyst had high thermal stability for CO2 methanation.
Si–OH (silanol sites) on the SBA-15 surface. The Ni-grafted SBA-15 catalyst suited CO2 methanation, resulting in higher CO2 conversion and methane selectivity than a NiO dispersed SBA-15 catalyst obtained by the conventional post synthesis method. The activation energy for CO2 methanation increased with a decreasing initial Ni amount used. The rate equation for CO2 methanation could be expressed as: r = kCCO20.64CH24.05, where C is the concentration. The Ni-grafted SBA-15 catalyst had high thermal stability for CO2 methanation.

 Please wait while we load your content...
Please wait while we load your content...