Impact of in vitro non-enzymatic glycation on biophysical and biochemical regimes of human serum albumin: relevance in diabetes associated complications
Abstract
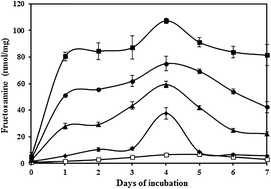
Early glycation involves attachment of glucose on ε-NH2 of lysine residues. Human serum albumin (HSA), a lysine rich protein, is highly prone to non-enzymatic glycation. In this study, the effect of different concentrations of glucose on HSA through early glycation was assessed by various physicochemical techniques. An early glycation product Amadori HSA was estimated by colorimetric method, nitro blue tetrazolium (NBT) and thiobarbituric acid assay (TBA). The conformational changes were confirmed by UV-visible spectroscopy, tryptophan fluorescence quenching, circular dichroism (CD), and sodium dodecyl sulphate poly acrylamide gel electrophoresis (SDS-PAGE). Biochemical alterations in Amadori-HSA were analyzed by NaBH4 reduction, increased carbonyl content and decrease in free lysine, arginine and sulfhydryl groups. Most studies have reported the structural changes in protein due to AGEs or Amadori protein at a particular glucose concentration; to the best of our knowledge, no reports have been published comparing the physicochemical changes in HSA due to early glycation within a range of glucose concentrations. In the present study, the investigations confirmed the structural and biochemical alterations in Amadori-HSA which is directly proportional to glucose concentration. This might interfere with the normal function of HSA and can contribute to the progression of diabetes and its associated complications. Amadori modified HSA might be used as a biomarker for early detection of diabetes and be beneficial in preventing the diabetic complications at early stages of diabetes. In this context, this study provides additional information about biochemical and biophysical changes occurring in Amadori-HSA within the glucose range upon early glycation.

 Please wait while we load your content...
Please wait while we load your content...