Zero-valent iron nanoparticles with sustained high reductive activity for carbon tetrachloride dechlorination†
Abstract
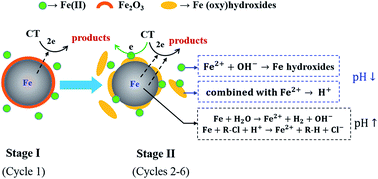
Zero-Valent Iron nanoparticles (nZVI) have been extensively applied for the reduction of various recalcitrant organic contaminants, but their reactivity usually declines over time due to the formation of passive iron oxides. In this study we observed a sustained reactivity of nZVI for the dechlorination of carbon tetrachloride (CT) in water during several consecutive reaction cycles. The dechlorination rate constants increased substantially in Cycle 2, then remained at a high level over several consecutive cycles, and ultimately declined in Cycle 7. In the entire process, the solution pH increased only slightly from 7.0 to 7.8, which was different from other unbuffered nZVI reduction systems reported before. Characterization of the particle surface morphology and composition revealed an important role of Fe oxyhydroxide formation in self-buffering the solution pH and sustaining a high nZVI reactivity. Our study provides new knowledge on the nZVI dechlorination process and may offer implications for extending the lifetime of nZVI in wastewater treatment and environmental remediation applications.

 Please wait while we load your content...
Please wait while we load your content...