Synthesis of new surfactant-like triazine-functionalized ligands for Pd-catalyzed Heck and Sonogashira reactions in water†
Abstract
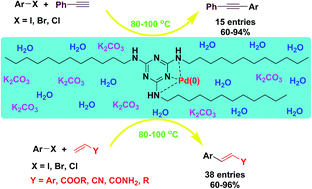
In this study, a novel class of ligands for palladium-catalyzed C–C coupling reactions in water is introduced. A range of triazine-functionalized ligands were synthesized using 2,4,6-trichloro-1,3,5-triazine (TCT) reagent. Among them, N2,N4,N6-tridodecyl-1,3,5-triazine-2,4,6-triamine (TDTAT) was found to be an efficient ligand for the Pd-catalyzed Heck and Sonogashira reactions in water, which acted as a green solvent. TEM analysis showed that in the presence of TDTAT in water at 80 °C, PdCl2 is converted to nanoparticles with an average size of ∼3 nm. The formed Pd-nanoparticles act as efficient catalytic species for C–C bond formation reactions in neat water. Under these conditions, Pd-catalyzed Heck and Sonogashira reactions are accomplished without the need for phosphine ligand. The generation of emulsion droplets (5–10 μm) containing Pd(0) nanoparticles would function as an effective reactor to accelerate the rate of the reaction in water media.

 Please wait while we load your content...
Please wait while we load your content...