Green and low-cost synthesis of PANI–TiO2 nanocomposite mesoporous films for photoelectrochemical water splitting†
Abstract
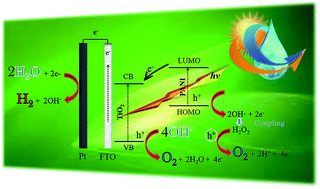
Among conductive polymers, polyaniline (PANI) has been widely used to improve electronic conductivity, solar energy transfer and photocatalytic activity of TiO2, due to its ease preparation and excellent environmental stability. In this study, a green and low-cost synthesis procedure was developed for the preparation of PANI–TiO2 nanocomposite films. A non-toxic and low-cost polymerization route, starting from aniline dimer and polystyrene sulphonate as emulsioning/doping agent in water, was employed to synthesize the conductive form of PANI (emeraldine salt, ES). Anatase TiO2 nanocrystalline mesoporous films were prepared by a novel and green sol–gel spin coating method, which employs titanium tetraisopropoxide, acetic acid and a nonionic surfactant (Tween 20) in excess of water, avoiding the use of flammable solvents. Uniform PANI–TiO2 composite films, containing PANI in either ES or pernigraniline base (PB) forms, i.e. PANI/TiO2 and PANIox/TiO2, respectively, were then prepared by a simple impregnation method. The films were characterized by means of XRD, ATR, FESEM and TEM techniques and their photocatalytic activity was assessed using them as photoelectrochemical water splitting photoanodes. Both PANI/TiO2 and PANIox/TiO2 showed an enhanced water oxidation efficiency under AM 1.5G simulated sunlight irradiation, reaching about 2 and 1.6 fold higher photocurrent densities, respectively, than a pure TiO2 nanoparticles film. They also demonstrated good stability after several hours of operation. UV-Vis spectrophotometry and IPCE analysis reveal the main role of PANI, in the system PANI/TiO2 for the PEC water oxidation, is as sensitizer of TiO2 in the UV light by significantly increasing charges separation, electrons transport and collected photoelectrons, indirectly contributing to the generation of O2. Indeed, PANI-ES photogenerated e− are transferred to the TiO2 conduction band while its h+ can react with OH− to produce OH radicals that generate H2O2, which can subsequently be photooxidized on the TiO2 NPs surface generating more O2 than such produced by the direct water oxidation on the TiO2 holes.

 Please wait while we load your content...
Please wait while we load your content...