Free oxoanion theory for BaMgAl10O17:Eu2+ structure decomposition during alkaline fusion process
Abstract
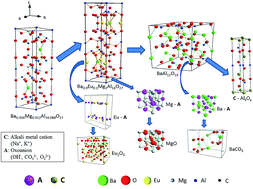
The alkaline fusion process is a useful pretreatment for rare earth elements (REEs) recycling from blue phosphor (BaMgAl10O17:Eu2+, BAM), but the lack of basic theory affects the further development of the alkaline fusion process. Different substances (KOH, NaOH, Ca(OH)2, NaCl, Na2CO3, and Na2O2) were chosen to react with BAM to explain the alkaline fusion process; only KOH, NaOH, Na2CO3, and Na2O2 can damage the BAM structure. The BAM alkaline fusion reactions share similar processes. Europium and magnesium ions were bonded with free oxoanion (OH−, CO32−, O22−) preferentially to escape from the BAM structure. The remaining structure of barium aluminate eventually decomposed into aluminate and BaCO3 in air. Cations (Na+, K+) were introduced to bond with the aluminate ions to maintain the charge balance of the reaction system. Free Oxoanion Theory (FOAT) was summarized to elucidate the structure decomposition process of BAM. The variation principle of determined lattice energy was in agreement with the FOAT analysis results. FOAT is very beneficial for the REEs recycling mechanism and alkaline fusion technology theory.

 Please wait while we load your content...
Please wait while we load your content...