Theoretical studies on gas-phase kinetics and mechanism of H-abstraction reaction from methanol by ClO and BrO radicals†
Abstract
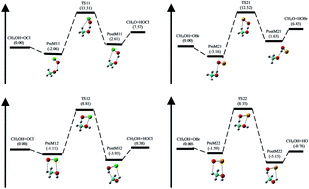
The gas-phase kinetics and mechanism of two channel hydrogen (H) abstraction reaction of methanol (CH3OH) by halogen monoxide (XO, X = Cl, Br) radical has been investigated using theoretical approach. The two H-abstraction channels followed are: hydroxyl H-atom or methyl H-atom of methanol (CH3OH). The geometry optimization and frequency calculations were performed at M06-2X method and cc-pVTZ basis set. Single point energy calculation of all the species were computed at high level CCSD(T)/cc-pVTZ theory on the M06-2X/cc-pVTZ optimized structure. Weak intermolecular pre-reactive and post-reactive complexes were located at the entrance and exit channel respectively on the potential energy surfaces of all the H-abstraction reactions. The rate constants (k) and branching ratio (ϕ) of all possible channels were calculated as a function of temperature for a wide range of temperature 200–2500 K. The rate constants were estimated using canonical variational transition state theory (CVT) combined with an Eckart tunneling correction and hindered rotor approximation for low frequency torsional modes. The rate constant calculation shows that the reaction for the H-abstraction from methyl group of CH3OH by XO radical leading to hydroxymethyl (CH2OH) radical is predominant over the hydroxyl group H-abstraction reaction forming methoxy (CH3O) radical. Arrhenius equation using three parameters was obtained by fitting the kinetic data for the two channels of H-abstraction by ClO and BrO radicals. The overall rate expression, in units of cm3 per molecule per s, with ClO radical is found to be kov1(T) = 3.92 × 10−19T1.63 exp(−2062/T) and with BrO radical it is kov2(T) = 1.53 × 10−20T2.41 exp(−2206/T) for the temperature range 200–2500 K.

 Please wait while we load your content...
Please wait while we load your content...