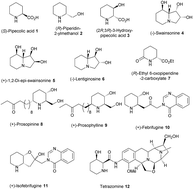
Enantioselective syntheses of (R)-pipecolic acid, (2R,3R)-3-hydroxypipecolic acid, β-(+)-conhydrine and (−)-swainsonine using an aziridine derived common chiral synthon†
Abstract
Concise total syntheses of (R)-pipecolic acid, (R)-ethyl-6-oxopipecolate, (2R,3R)-3-hydroxypipecolic acid and formal syntheses of β-(+)-conhydrine, (−)-lentiginosine, (−)-swainsonine and 1,2-di-epi-swainsonine have been accomplished starting from a common chiral synthon. The present strategy employs regioselective aziridine ring opening, Wittig olefination and RCM as the key chemical transformations.

 Please wait while we load your content...
Please wait while we load your content...